Part 1: Prepare Tubes
|
Purpose: Sterilize your workspace to reduce the chance of contamination. Set up your control and pFLO transformation.
|
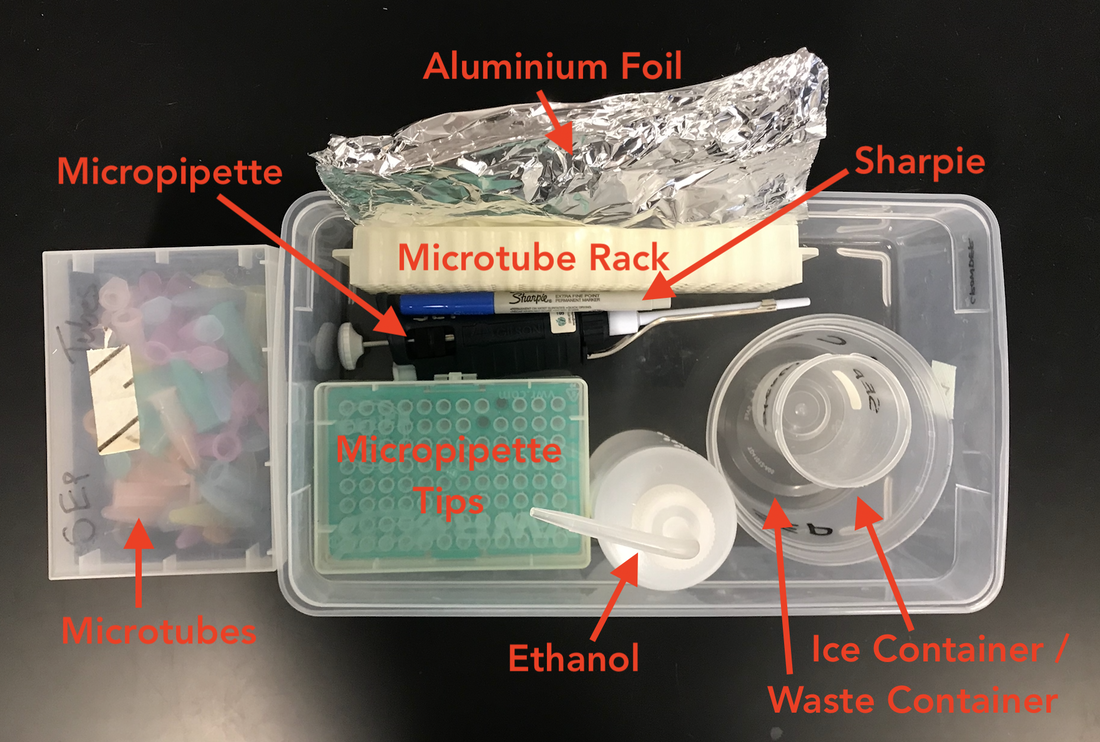
Tubes are located at your lab station.
Calcium chloride and micropipettes are located on the table near the chemical shower. Ice is located in the back of the room. Your ice container is at your lab station. |
Part 2: Add Bacteria to Tubes
|
Purpose: Each colony on the stock plate originates from a single “mother” cell. Selecting a single colony ensures that all the bacteria you’ll be growing and transforming are genetically alike. The plates we will be using were made from a single colony so all the bacteria on the plates should be genetically alike.
|
Bacteria are located on the table by the chemical shower. Open the bacteria spreader at the pointy end you do not contaminate the round end when you pull it out of the sleeve. Sterile transfer pipettes are at your lab station. |
Part 3: Add Plasmid to Bacteria
|
Purpose: Transform bacteria with the pFLO plasmid to express antibiotic resistance and the gene of interest. There are three different types of pFLO plasmid. Each is a different color. Chose one color for your group.
|
The plasmid is in a freezer box on the table near the chemical shower.
p20 micropipettes and tips are on the table with the plasmid. Sterile water is on the table by the chemical shower. Use the timer on your phone or Chromebook. |
Part 4: Heatshock
|
Purpose: The CaCl2 and heatshock alter the cell membrane of the bacteria so that foreign DNA can pass through more easily. These cells are called “competent”. This is an essential step for successful transformation.
|
There are two hot water baths near the goggles. Make sure you are using the 42 degree hot water bath for the heat shock.
Use a foam floaty to keep your tubes upright. Make sure the tubes are closed tightly before putting them in the water bath. The water is not hot enough to burn you but don't touch it more than you need to. The p1000, tips, and Luria Broth are located on the table near the chemical shower. |
Part 5: Plating Cells
|
Purpose: To spread individual cells evenly over the surface of the plate resulting in visible colonies after incubation.
|
You will label plates during the recovery step in part 4. Ask you teacher to help you label the plates if the class is not ready to do it as a group.
Write small and on the bottom of the plates. Keep the LB/amp plate under tinfoil to protect it from the light. There is a p50/p100 at your lab station. |
Clean up
Proudly powered by Weebly