Transport across the cell membrane
|
|
|
|
Diffusion
Small molecules can pass through the cell membrane through a process called diffusion. Diffusion is the movement of molecules from an area where there is a higher concentration(larger amount) of the substance to an area where there is a lower concentration (lower amount) of the substance. The amount of a substance in relation to the total volume is the concentration. During diffusion, molecules are said to flow down their concentration gradient, flowing from an area of high concentration to an area of low concentration. Molecules flowing down a concentration gradient is a natural process and does not require energy. Diffusion can occur across a semipermeable membrane, such as the cell membrane, as long as a concentration gradient exists. Molecules will continue to flow in this manner until equilibrium is reached. At equilibrium, there is no longer an area of high concentration or low concentration, and molecules flow equally in both directions across the semipermeable membrane. At equilibrium, equal amounts of a molecule are entering and leaving a cell. |
|
|
|
|
|
Facilitated Diffusion
What happens if a substance needs assistance to move across or through the plasma membrane? Facilitated diffusion is the diffusion of solutes through transport proteins in the plasma membrane. Facilitated diffusion is a type of passive transport. Even though facilitated diffusion involves transport proteins, it is still passive transport because the solute is moving down the concentration gradient.
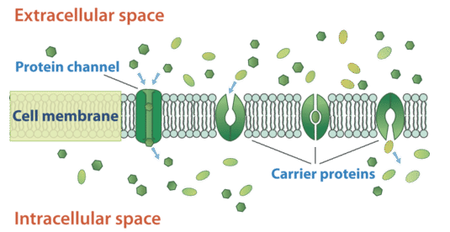
Small nonpolar molecules can easily diffuse across the cell membrane. However, due to the nature of the lipids that make up cell membranes, polar molecules (such as water) and ions cannot do so. Instead, they diffuse across the membrane through transport proteins. A transport protein completely spans the membrane, and allows certain molecules or ions to diffuse across the membrane. Channel proteins, gated channel proteins, and carrier proteins are three types of transport proteins that are involved in facilitated diffusion.
A channel protein, a type of transport protein, acts like a pore in the membrane that lets water molecules or small ions through quickly. Water channel proteins (aquaporins) allow water to diffuse across the membrane at a very fast rate. Ion channel proteins allow ions to diffuse across the membrane.
A carrier protein is a transport protein that is specific for an ion, molecule, or group of substances. Carrier proteins "carry" the ion or molecule across the membrane by changing shape after the binding of the ion or molecule. Carrier proteins are involved in passive and active transport.
What happens if a substance needs assistance to move across or through the plasma membrane? Facilitated diffusion is the diffusion of solutes through transport proteins in the plasma membrane. Facilitated diffusion is a type of passive transport. Even though facilitated diffusion involves transport proteins, it is still passive transport because the solute is moving down the concentration gradient.
Small nonpolar molecules can easily diffuse across the cell membrane. However, due to the nature of the lipids that make up cell membranes, polar molecules (such as water) and ions cannot do so. Instead, they diffuse across the membrane through transport proteins. A transport protein completely spans the membrane, and allows certain molecules or ions to diffuse across the membrane. Channel proteins, gated channel proteins, and carrier proteins are three types of transport proteins that are involved in facilitated diffusion.
A channel protein, a type of transport protein, acts like a pore in the membrane that lets water molecules or small ions through quickly. Water channel proteins (aquaporins) allow water to diffuse across the membrane at a very fast rate. Ion channel proteins allow ions to diffuse across the membrane.
A carrier protein is a transport protein that is specific for an ion, molecule, or group of substances. Carrier proteins "carry" the ion or molecule across the membrane by changing shape after the binding of the ion or molecule. Carrier proteins are involved in passive and active transport.
|
Credit: Hana Zavadska, based on image by Mariana Ruiz Villarreal (http://commons.wikimedia.org/wiki/File:Scheme_facilitated_diffusion_in_cell_membrane-en.svg)
Source: CK-12 Foundation License: CC BY-NC 3.0 |
This text is adapted under a Creative Commons 4.0 license. You can find the original source here.
|
Osmosis
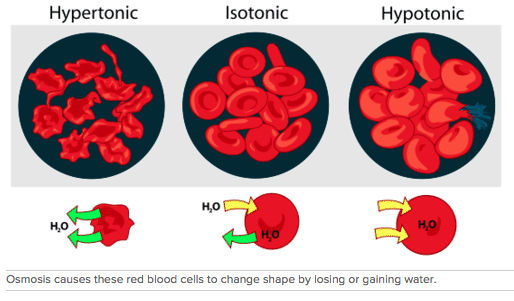
The diffusion of water across a membrane because of a difference in concentration is called osmosis. Water is attracted to the molecules dissolved in water. In osmosis, the water moves rather than the dissolved molecules. Because water is attracted to the dissolved molecules, the water will move from areas of low dissolved molecule concentration to areas that have higher dissolved molecule concentration. Let's explore three different situations and analyze the flow of water.
|
|
|
|
|
Applications of Osmosis
How do marine animals keep their cells from shrinking? How do you keep your blood cells from bursting? Both of these questions have to do with the cell membrane and osmosis. Marine animals live in salt water, which is a hypertonic environment; there is more salt in the water than in their cells. To prevent losing too much water from their bodies, these animals intake large quantities of salt water and then secrete the excess salt. Red blood cells can be kept from bursting or shriveling if put in a solution that is isotonic to the blood cells. If the blood cells were put in pure water, the solution would be hypotonic to the blood cells, so water would enter the blood cells, and they would swell and burst
How do marine animals keep their cells from shrinking? How do you keep your blood cells from bursting? Both of these questions have to do with the cell membrane and osmosis. Marine animals live in salt water, which is a hypertonic environment; there is more salt in the water than in their cells. To prevent losing too much water from their bodies, these animals intake large quantities of salt water and then secrete the excess salt. Red blood cells can be kept from bursting or shriveling if put in a solution that is isotonic to the blood cells. If the blood cells were put in pure water, the solution would be hypotonic to the blood cells, so water would enter the blood cells, and they would swell and burst
|
Credit: Mariana Ruiz Villarreal (LadyofHats)
Source: http://commons.wikimedia.org/wiki/File:Osmotic_pressure_on_blood_cells_diagram.svg License: Public Domain This text is adapted under a Creative Commons 4.0 license. You can find the original source here.
|
|
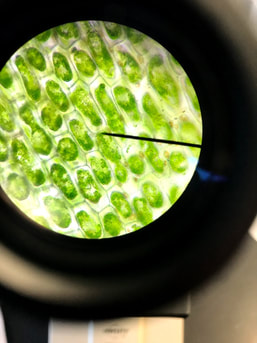
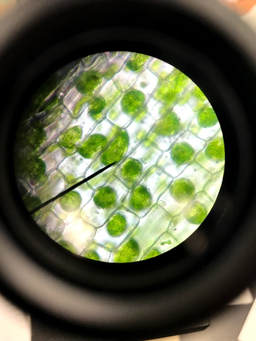
These pictures are of Elodea under a microscope. Both are viewed at 400x. In the picture on the left the elodea has been placed in a 5% salt solution. In the photo on the right the elodea has been placed in a 10% salt solution.
In these photos, salt water outside the cell has caused water to move out of the cell. As water moves out of the cell the cell membrane shrivels up and the chloroplasts are forced into a smaller and smaller space, clumping them together in the center of the cell. |
5% salt solution
|
10% salt solution
|
|
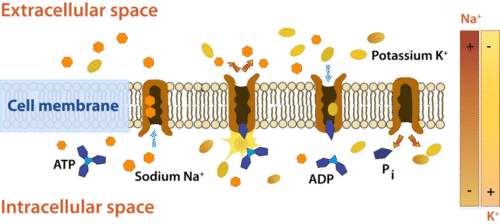
Active Transport
During active transport, molecules move from an area of low concentration to an area of high concentration. This is the opposite of diffusion, and these molecules are said to flow against their concentration gradient. Active transport is called "active" because this type of transport requires energy to move molecules. As molecules are moving against their concentration gradients, active transport cannot occur without assistance. A carrier protein is always required in this process. Like facilitated diffusion, a protein in the membrane carries the molecules across the membrane, except this protein moves the molecules from a low concentration to a high concentration. These proteins are often called "pumps" because they use energy to pump the molecules across the membrane. There are many cells in your body that use pumps to move molecules. For example, your nerve cells (neurons) would not send messages to your brain unless you had protein pumps moving molecules by active transport. |
|
|
The sodium-potassium pump moves sodium ions to the outside of the cell and potassium ions to the inside of the cell, areas where these ions are already highly concentrated. ATP is required for the protein to change shape. ATP is converted into ADP (adenosine diphosphate) during active transport.
|
Proudly powered by Weebly