|
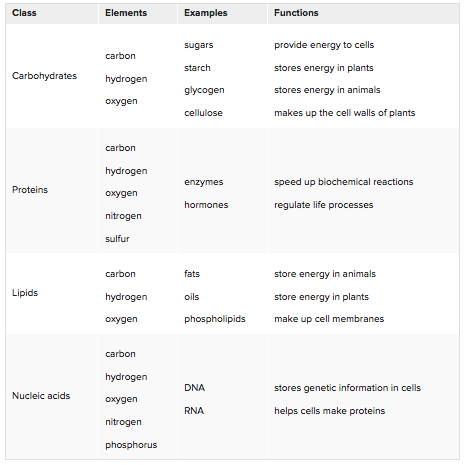
Classes of Biochemical Compounds
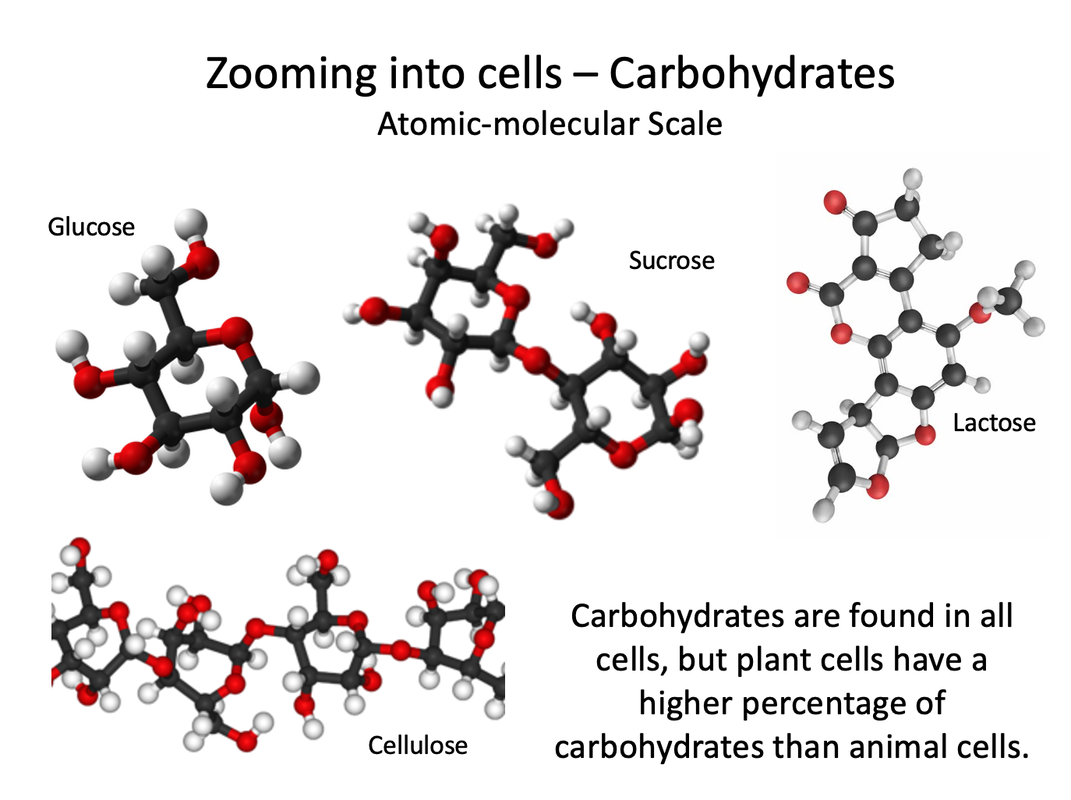
Biochemical compounds make up the cells and tissues of living things. They are also involved in all life processes. Given their diversity of functions, it’s not surprising that there are millions of different biochemical compounds. Even so, all biochemical compounds can be grouped into just four main classes: carbohydrates, proteins, lipids, and nucleic acids. |
|
|
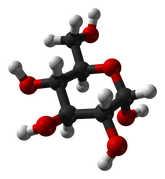
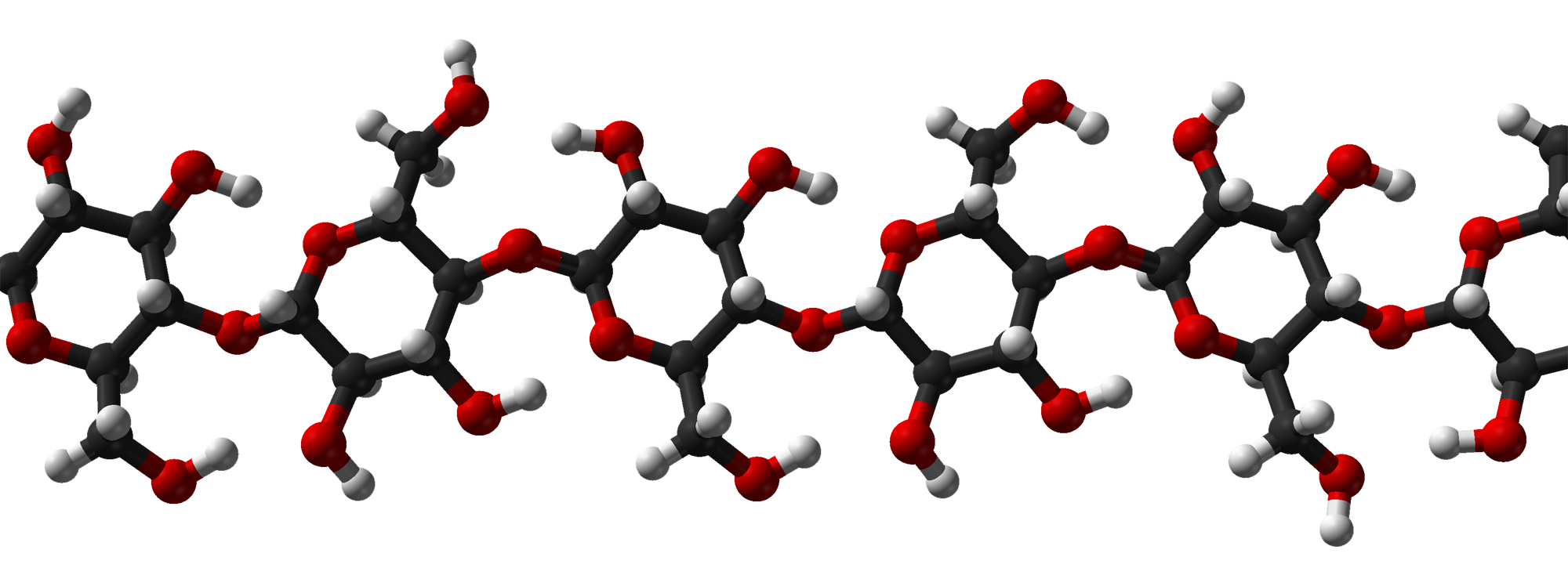
Cellulose is another complex carbohydrate found in plants that is a polymer of glucose. Cellulose molecules bundle together to form long, tough fibers. Cellulose is the most abundant biochemical compound. It makes up the cell walls of plants and gives support to stems and tree trunks
|
|
Proteins
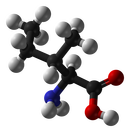
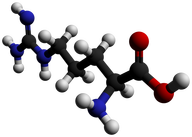
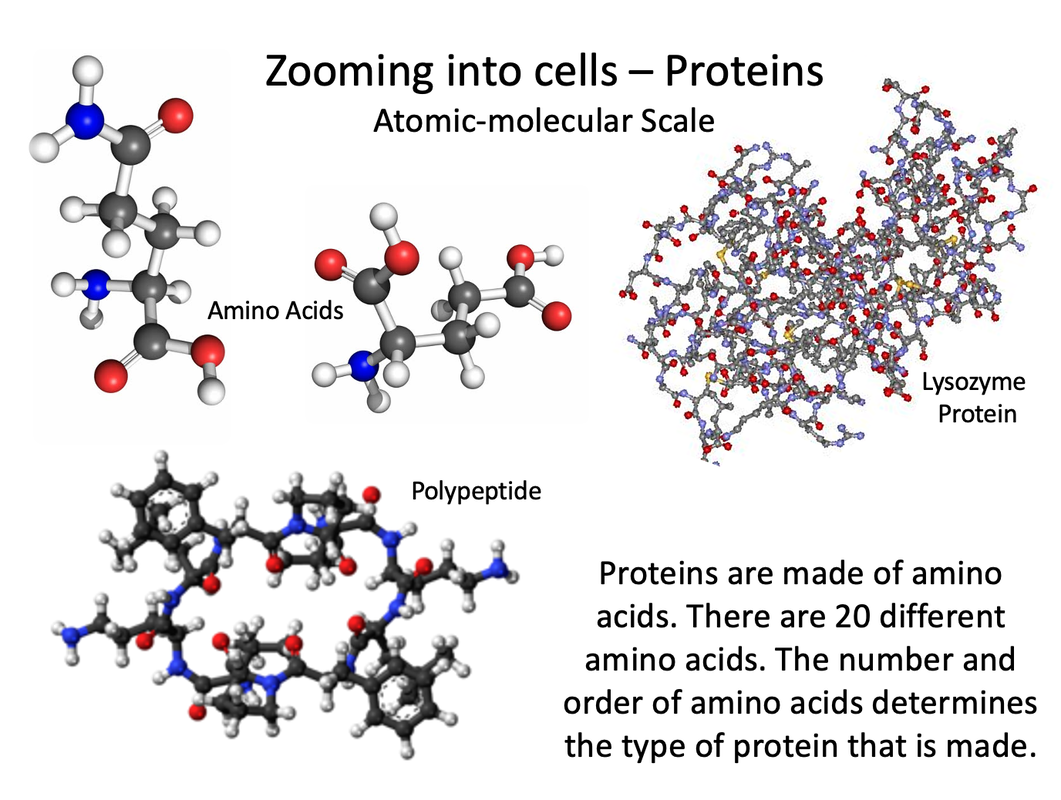
Proteins are biochemical compounds that consist of one or more chains of small molecules called amino acids. Amino acids are the monomers of proteins. There are only about 20 different amino acids. The sequence of amino acids in chains and the number of chains in a protein determine the protein’s shape. Shapes may be very complex. The shape of a protein determines its function. Proteins have many different functions. For example, proteins:
|
|
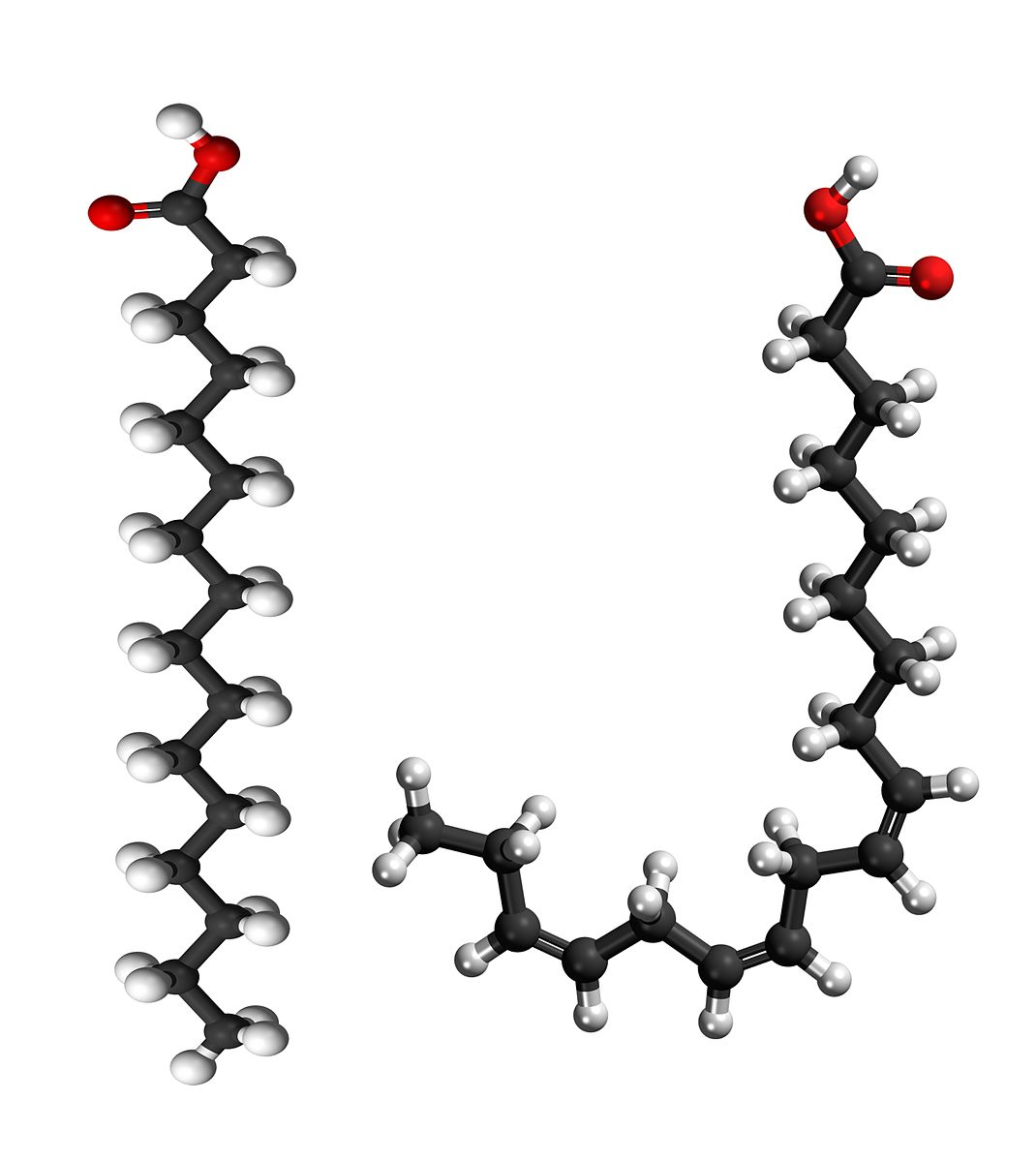
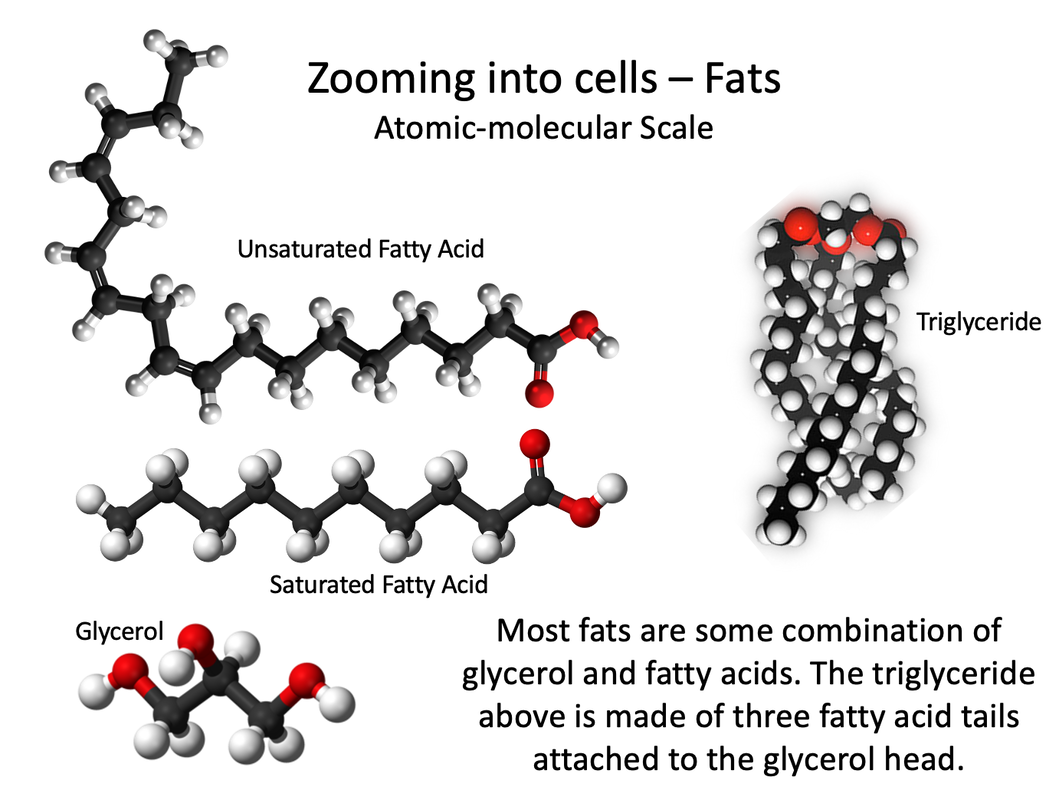
Lipids
Lipids are biochemical compounds that living things use to store energy and make cell membranes. Types of lipids include fats, oils, and phospholipids.
|
|
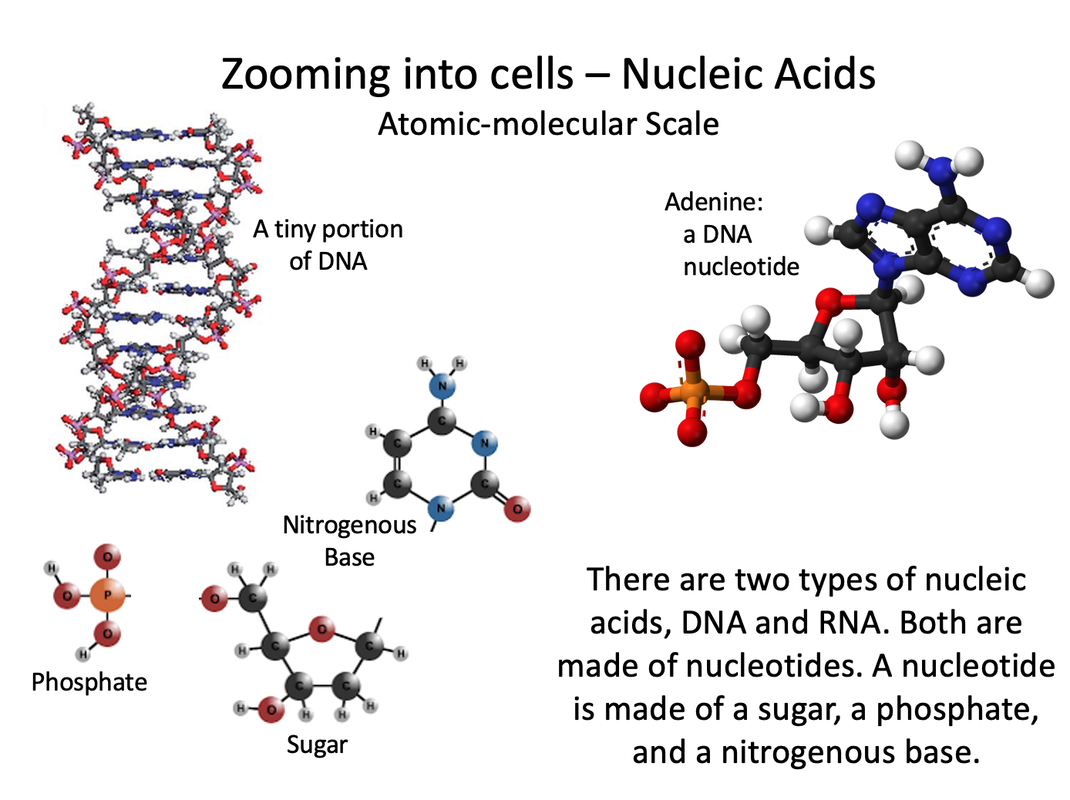
Nucleic Acids
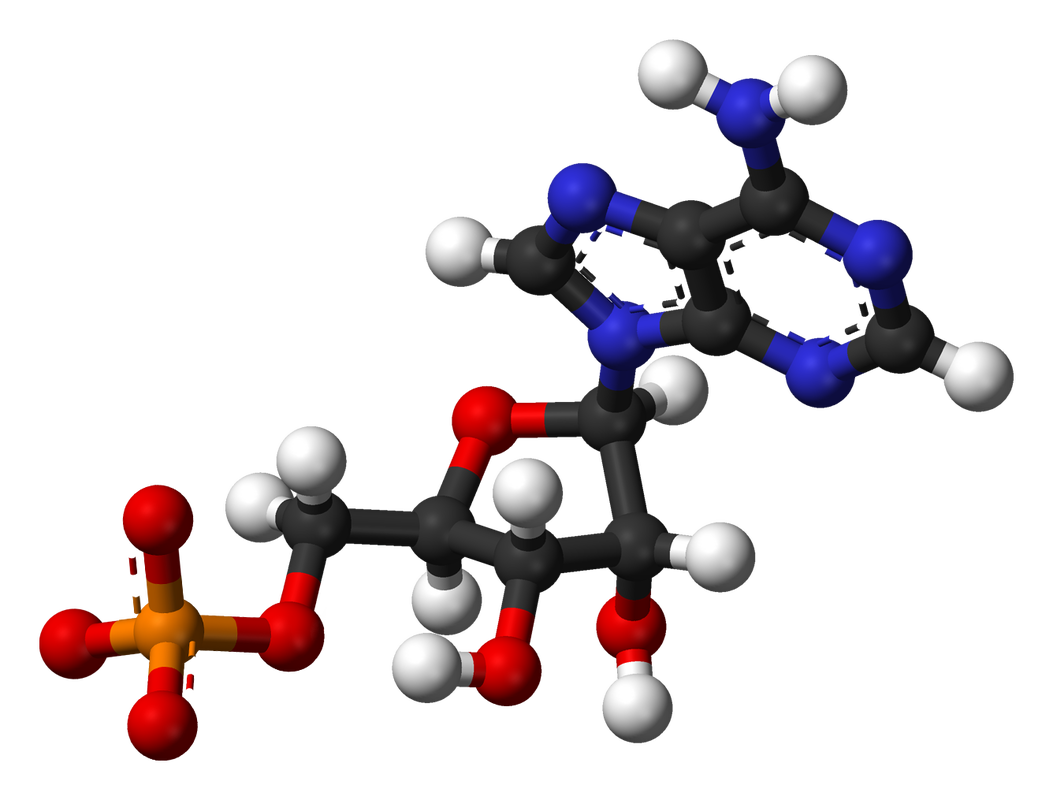
Nucleic acids are biochemical compounds that include RNA (ribonucleic acid) and DNA (deoxyribonucleic acid). Nucleic acids consist of chains of small molecules called nucleotides. Nucleotides are the monomers of nucleic acids. Each nucleotide consists of:
DNA stores genetic information in the cells of all living things. It contains the genetic code. This is the code that instructs cells how to make proteins. The instructions are encoded in the sequence of nitrogen bases in DNA’s nucleotide chains. RNA copies and interprets the genetic code in DNA. |
|
|
|
Proudly powered by Weebly