|
Classes of Biochemical Compounds
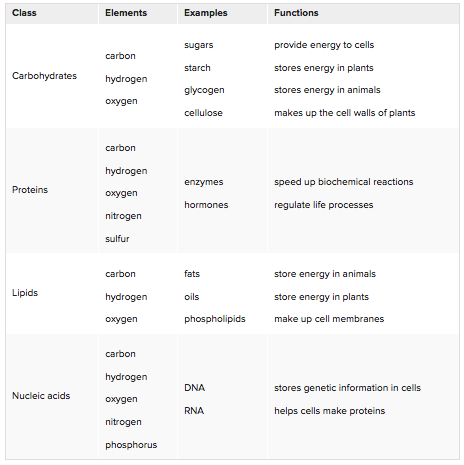
Biochemical compounds make up the cells and tissues of living things. They are also involved in all life processes. Given their diversity of functions, it’s not surprising that there are millions of different biochemical compounds. Even so, all biochemical compounds can be grouped into just four main classes: carbohydrates, proteins, lipids, and nucleic acids. |
|
Carbohydrates
Carbohydrates are biochemical compounds that include sugar, starch, glycogen, and cellulose. Sugars are simple carbohydrates with relatively small molecules. Glucose is the smallest of all the sugar molecules with its chemical formula of C6H12O6. This means that a molecule of glucose contains 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen. Plants and some other organisms make glucose in the process of photosynthesis. Living things that cannot make glucose can obtain it by consuming plants or organisms that consume plants. Starches are complex carbohydrates. They are polymers of glucose. Starches contain hundreds of glucose monomers. Plants make starches to store extra glucose. Consumers can get starches by eating plants. Our digestive system breaks down starches to sugar, which our cells use for energy. Like other animals, we store any extra glucose as the complex carbohydrate called glycogen. Glycogen is also a polymer of glucose. |
Cellulose is another complex carbohydrate found in plants that is a polymer of glucose. Cellulose molecules bundle together to form long, tough fibers. Cellulose is the most abundant biochemical compound. It makes up the cell walls of plants and gives support to stems and tree trunks.
Proteins
Proteins are biochemical compounds that consist of one or more chains of small molecules called amino acids. Amino acids are the monomers of proteins. There are only about 20 different amino acids. The sequence of amino acids in chains and the number of chains in a protein determine the protein’s shape. Shapes may be very complex. The shape of a protein determines its function. Proteins have many different functions. For example, proteins:
Lipids
Lipids are biochemical compounds that living things use to store energy and make cell membranes. Types of lipids include fats, oils, and phospholipids.
Nucleic Acids
Nucleic acids are biochemical compounds that include RNA (ribonucleic acid) and DNA (deoxyribonucleic acid). Nucleic acids consist of chains of small molecules called nucleotides. Nucleotides are the monomers of nucleic acids.
Each nucleotide consists of:
DNA stores genetic information in the cells of all living things. It contains the genetic code. This is the code that instructs cells how to make proteins. The instructions are encoded in the sequence of nitrogen bases in DNA’s nucleotide chains. RNA copies and interprets the genetic code in DNA.
Proteins
Proteins are biochemical compounds that consist of one or more chains of small molecules called amino acids. Amino acids are the monomers of proteins. There are only about 20 different amino acids. The sequence of amino acids in chains and the number of chains in a protein determine the protein’s shape. Shapes may be very complex. The shape of a protein determines its function. Proteins have many different functions. For example, proteins:
- make up muscle tissues.
- speed up chemical reactions in cells.
- regulate life processes.
- help defend against infections.
- transport materials around the body in the blood.
Lipids
Lipids are biochemical compounds that living things use to store energy and make cell membranes. Types of lipids include fats, oils, and phospholipids.
- Fats are solid lipids that animals use to store energy. Examples of fats include butter and the fat in meat.
- Oils are liquid lipids that plants use to store energy. Examples of oils include olive oil and corn oil.
- Phospholipids contain the element phosphorus. They make up the cell membranes of living things.
- In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. In other words, the carbon atoms are saturated with hydrogen. Saturated fatty acids are found in fats.
- In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they share double bonds with other carbon atoms. Unsaturated fatty acids are found in oils.
Nucleic Acids
Nucleic acids are biochemical compounds that include RNA (ribonucleic acid) and DNA (deoxyribonucleic acid). Nucleic acids consist of chains of small molecules called nucleotides. Nucleotides are the monomers of nucleic acids.
Each nucleotide consists of:
- a phosphate group, which contains phosphorus and oxygen.
- a sugar, which is deoxyribose in DNA and ribose in RNA.
- one of four nitrogen-containing bases. (A base is a compound that is not neither acidic nor neutral.) In DNA, the bases are adenine, thymine, guanine, and cytosine. RNA has the base uracil instead of thymine, but the other three bases are the same.
DNA stores genetic information in the cells of all living things. It contains the genetic code. This is the code that instructs cells how to make proteins. The instructions are encoded in the sequence of nitrogen bases in DNA’s nucleotide chains. RNA copies and interprets the genetic code in DNA.
|
Biochemical Reactions
The student athlete, shown to the right, is practically flying down the track! Running takes a lot of energy. But you don’t have to run a race to use energy. All living things need energy all the time just to stay alive. The energy is produced in chemical reactions. A chemical reaction is a process in which some substances, called reactants, change chemically into different substances, called products. Reactants and products may be elements or compounds. Chemical reactions that take place inside living things are called biochemical reactions. Living things depend on biochemical reactions for more than just energy. Every function and structure of a living organism depends on thousands of biochemical reactions taking place in each cell. |
Metabolism
The sum of all of an organism’s biochemical reactions is called metabolism. Biochemical reactions of metabolism can be divided into two general categories: catabolic reactions and anabolic reactions.
The sum of all of an organism’s biochemical reactions is called metabolism. Biochemical reactions of metabolism can be divided into two general categories: catabolic reactions and anabolic reactions.
- Anabolic reactions involve forming bonds. Smaller molecules combine to form larger ones. These reactions require energy. For example, it takes energy to build starches from sugars.
- Catabolic reactions involve breaking bonds. Larger molecules break down to form smaller ones. These reactions release energy. For example, energy is released when starches break down to sugars.
|
Photosynthesis and Cellular Respiration
Some of the most important biochemical reactions are the reactions involved in photosynthesis and cellular respiration.
|
Lesson Summary
- All living things are made of matter. Matter can consist of pure substances called elements or of combinations of elements called chemical compounds. Atoms are the smallest particles of elements. Molecules are the smallest particles of compounds.
- Biochemical compounds are carbon-based compounds that make up living organisms. There are four main classes of biochemical compounds: carbohydrates, proteins, lipids, and nucleic acids. The classes have different structures and functions.
- Life depends on biochemical reactions constantly taking place inside cells. Metabolism is the sum of all the biochemical reactions in an organism. It includes anabolic reactions that build up molecules and catabolic reactions that break down molecules. Enzymes speed up biochemical reactions.
This text is adapted from CK12.com, under a Creative Commons 4.0 license. You can find the original source here.
Proudly powered by Weebly